Over 350 million Peripheral IV Catheters are sold in the US each year, with over 90% of hospital patients getting vital care through them.
Yet vascular access device failure is pervasive. Patients often complain of multiple catheter insertion attempts, and published data reveal up to 53% of PIVCs fail before therapy ends.
Traditional IV Catheter designs often lead to blood exposure, vein damage, and fluid leakage at the vein entry site, which can all contribute to device failure and patient harm.
I-V Access Technology (IVAT) has created two innovative design platforms: VenaValve® – Our BC-X® Multi-Access Blood Control Valve, and VenaSave® – Our unique, three layer Vein Preservation system. Both are designed to reduce the common problems with IV Access.


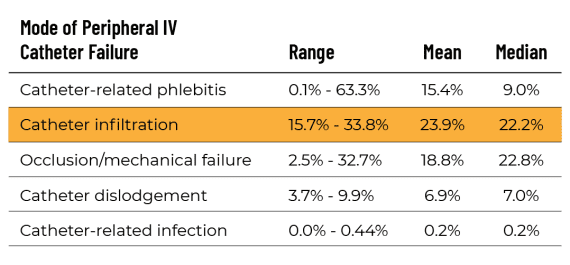
Journal of Infusion Nursing,“Accepted But Unacceptable:
Peripheral IV Catheter Failure,”June 2015 *
Minimize blood exposure with our BC-X® multiple-access blood control technology
Our BC-X® technology is designed to have the flexibility to fit into current or new IV catheters
Minimize blood exposure with our BC-X® multiple-access blood control technology
Improve venous seal and reduce fluid leakage with smaller incision
Integrate our industry-leading components into your products

Join our efforts to combat the pervasive problems of IV failure
* Helm RE, Klausner JD, Klemperer JD, et al. Accepted but unacceptable: peripheral IV catheter failure. J Infus Nurs 2015;38:189–203
I-V Access Technology, Inc.
88 Perry Street #800
San Francisco, CA 94107
IVAT, BC-X, VenaValve and VenaSave are registered trademarks of I-V Access Technology.
The BC-X Multiple Access Blood Control Valve and IVAT’s Three Layer Safety Design are components and have not been cleared by the FDA for commercial sale in the US or any other region.
©2023 I-V Access Technology, Inc. All rights reserved.